Biopharmaceutical manufacturing relies on the skillful application of aseptic techniques to ensure the manufacture and distribution of sterile drug products. Due to the chemically fragile nature of heat-labile drugs, including many biologics and proteinaceous molecules, aseptic processing is utilized to manufacture product that is typically delivered by injection directly into a patient’s bloodstream. Contamination of these drug products via the introduction of microorganisms, endotoxins or particulates leads to product failure, product destruction and the potential for illness or death of patients and consumers. In our aseptic processing courses, students experience hands-on training in aseptic techniques in a simulated CGMP biomanufacturing environment to ready them for various careers in the biopharmaceutical industry.

Aseptic Processing Concepts
Aseptic processing is utilized to prevent contamination and manufacture sterile products to ensure patient safety.…

Aseptic Processing I
Are you new to the pharmaceutical or life science sectors? Need to brush up your skills? Learn and practice the…

Aseptic Processing II
Build on the foundation you constructed in Aseptic Processing Level I with this course, a hands-on experience in which…

BioCertification Review
All students who complete the BioWork Certificate Program successfully are eligible to take the certification exam.…

Cell and Tissue Culture Techniques
This course provides students with a sound, practical, and theoretical knowledge of key techniques to perform cell…

Cleanroom Gowning Concepts
Aseptic gowning is a system of donning apparel to prevent the contamination of aseptic processing areas. The Cleanroom…
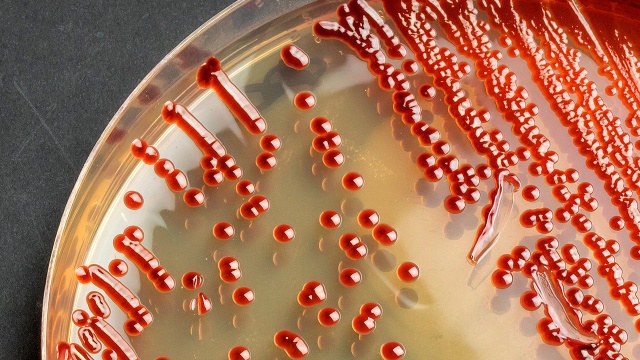
Environmental Monitoring Program for Aseptic Processing
An Environmental Monitoring (EM) program provides meaningful information on the quality of the aseptic…

Introduction to Cell and Gene Therapy
Cell and Gene Therapy is a technique that modifies a person's genes to treat or cure disease. This course will focus on…