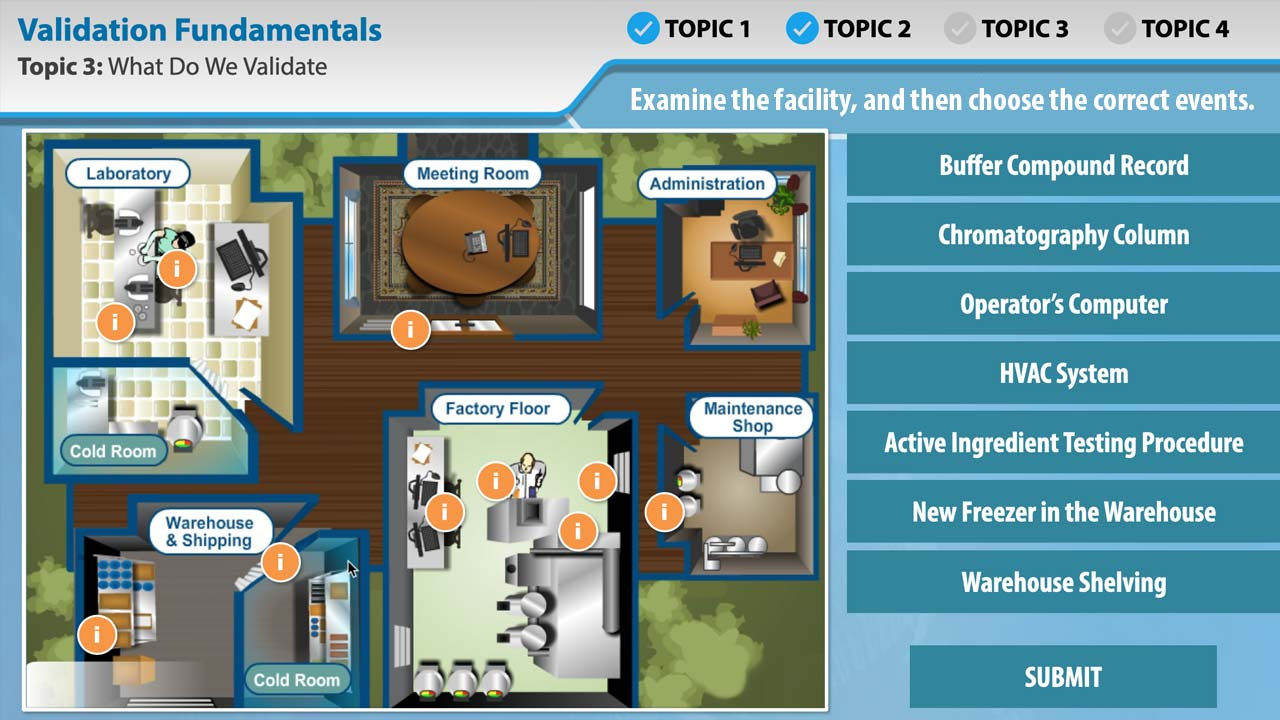
Overview
In this short eLearning course, you'll learn the fundamentals of process and equipment validation in the manufacture of pharmaceuticals. Explore the history of validation, what validation means, what must be validated, and when it must be validated.