Overview
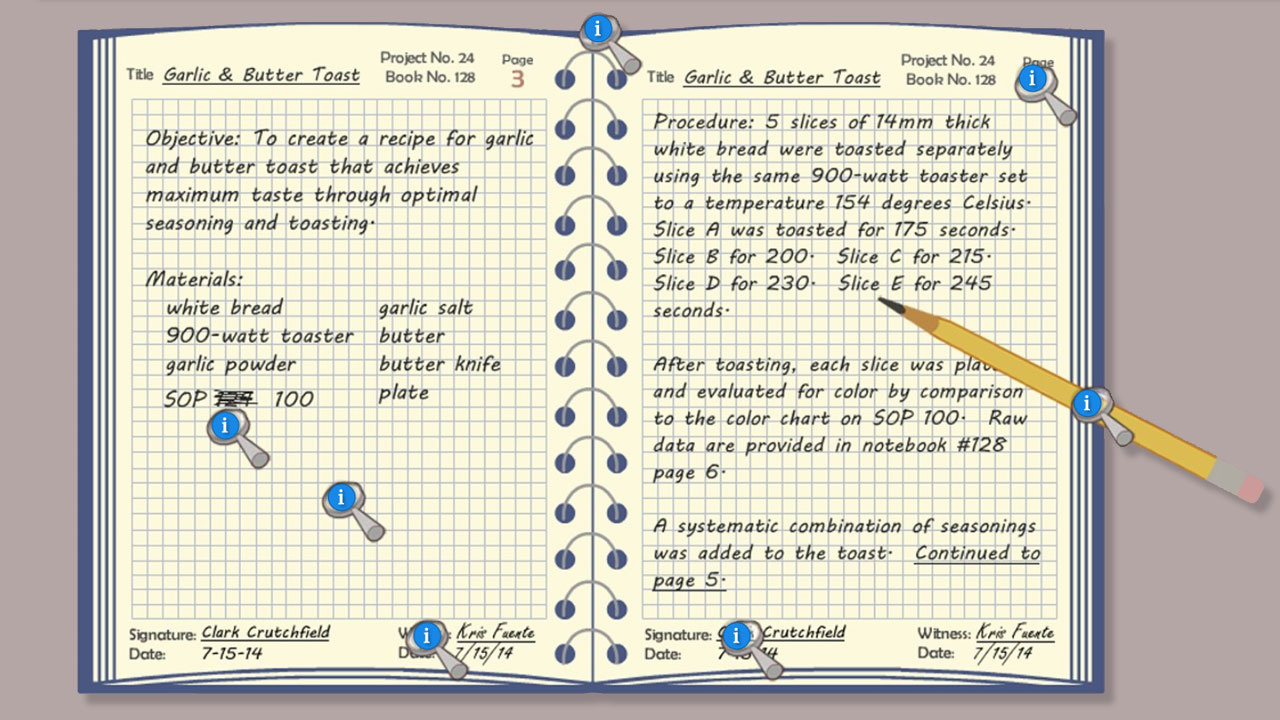
You’re the newest member of the Quality Assurance department at CATT – the Center for the Advancement of Toast Technology. Your mission is to audit the company and determine if its SOPs, notebooks, equipment, personnel, reagent labels, and test animals are GLP compliant.
Objectives
Upon completion of this exercise, you will be able to:
- Explain the importance of Standard Operating Procedures
- Describe the relevance of qualified personnel
- Recognize common chemical safety hazards
- Define the reasons for using a laboratory notebook
- Explain the use of proper documentation