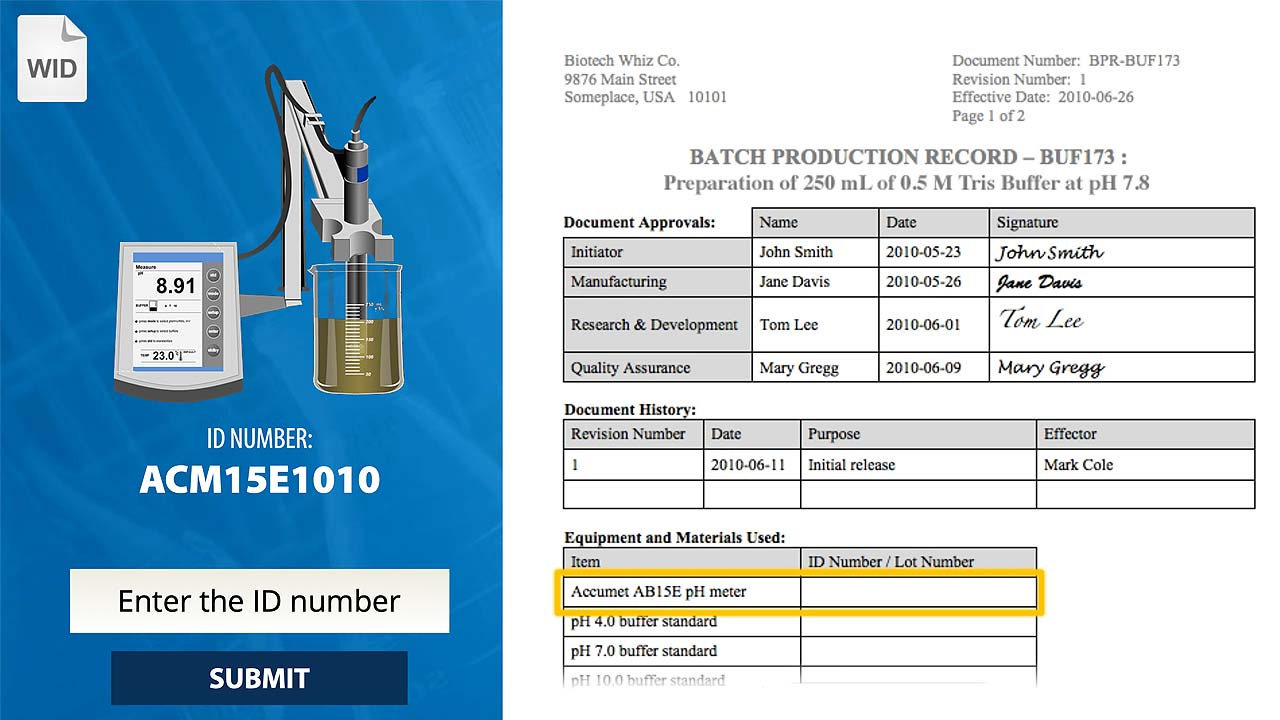
Overview
Documentation is a critical part of any manufacturing process. Every manufacturing step is controlled with a set of documents that define and record production activities in order to ensure a safe and effective product.
Objectives
Upon completion of this exercise, you will be able to:
- Define the common types of documents
- Recognize the difference between a WID, SOP, and BPR
- Explain the importance of each type of document
- Complete a simulated BPR by following the SOP and WID