Overview
These eLearning resources equip any employee who writes, edits, or compiles documents used in manufacturing FDA regulated products, including:
- Pharmaceutical Production Associates
- Production Managers
- Quality Managers
- Management
Good Documentation Practices (GDP)

Batch Documents
eLearning
In this interactive exercise, you will learn the importance of batch documents by reviewing the core values that define them, exploring their importance to the manufacturing process, and identifying and correcting the most common types of errors encountered in batch documentation.
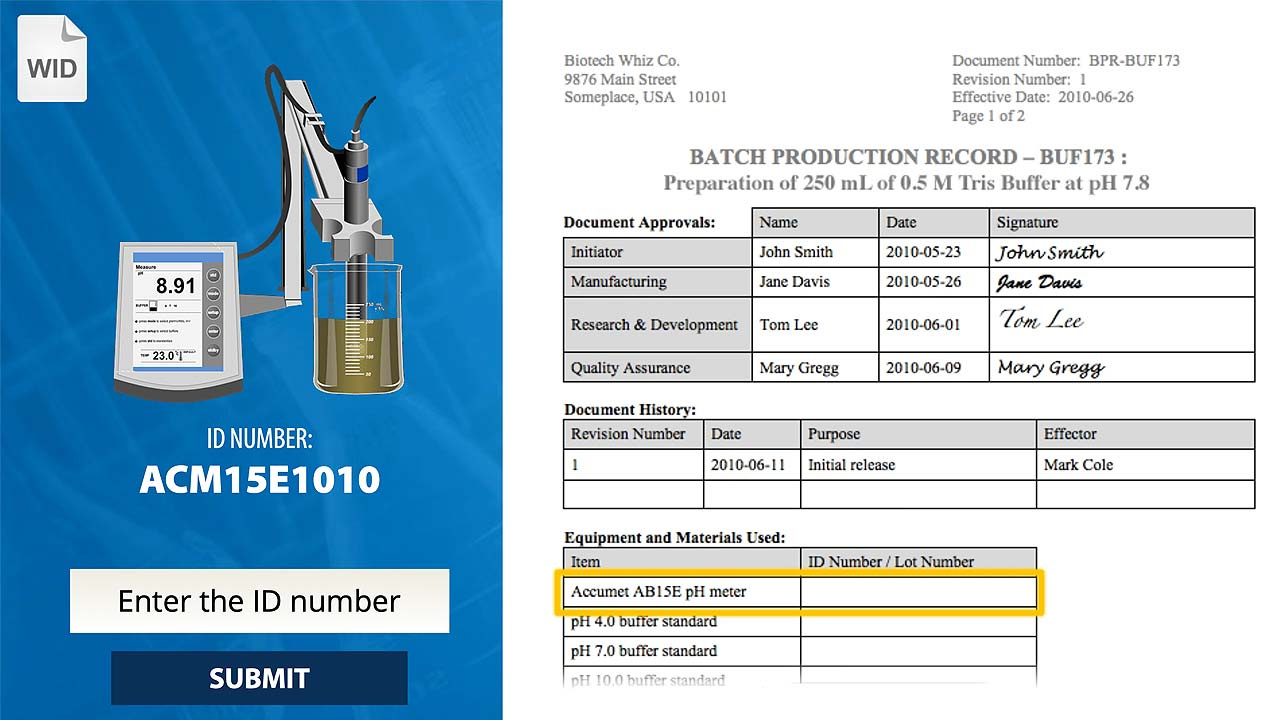
Deliberate Documentation
eLearning
Documentation is a critical part of any manufacturing process. Every manufacturing step is controlled with a set of documents that define and record production activities in order to ensure a safe and effective product.
